- GST NO. : 27AAJCV9819Q1Z6
- Send Email
+91-8149932782 Call / WhatsApp |
Drop us a line |
| Business Type | Exporter, Supplier, Trader |
| Prescription/Non-Prescription | Prescription |
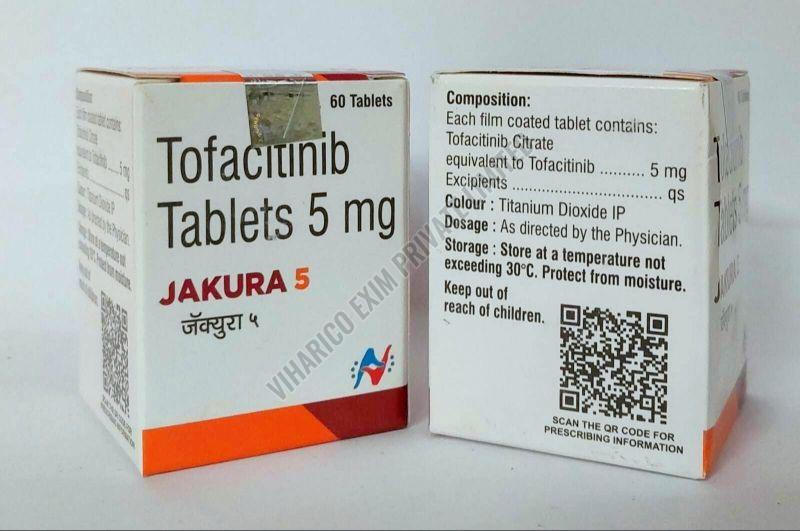
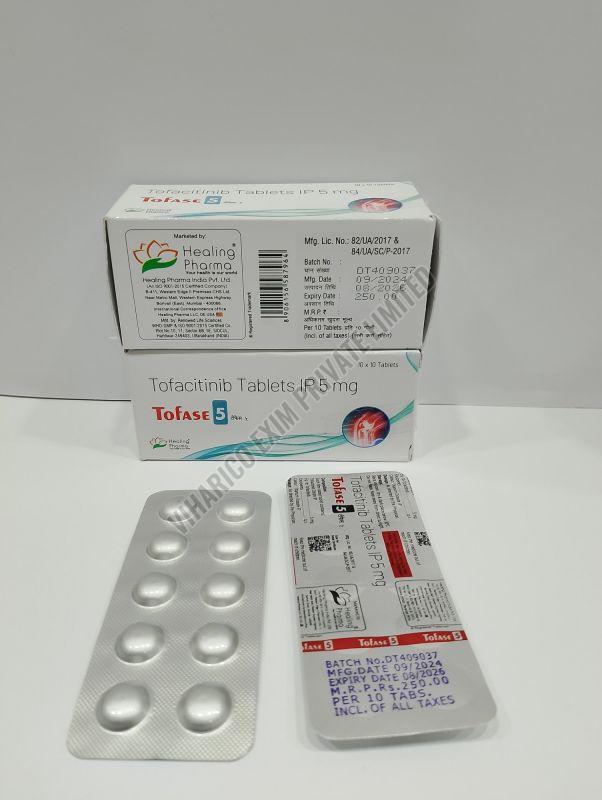
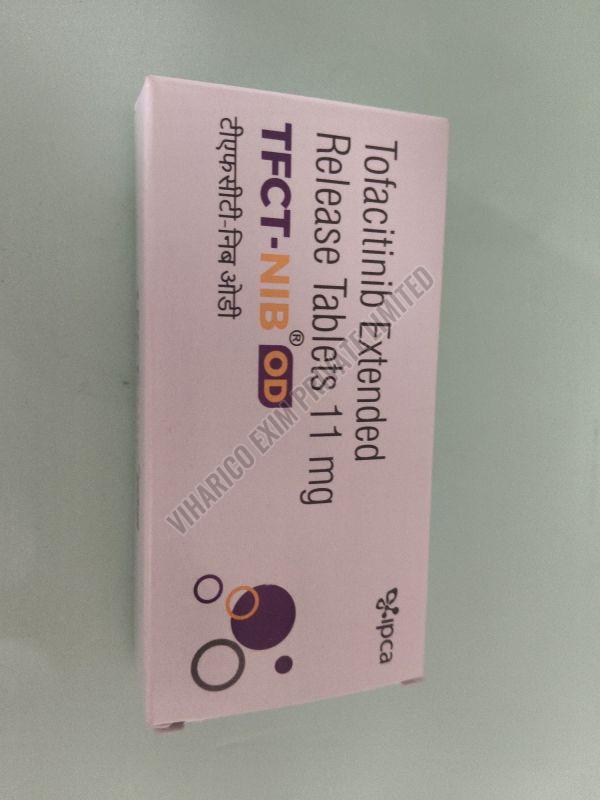
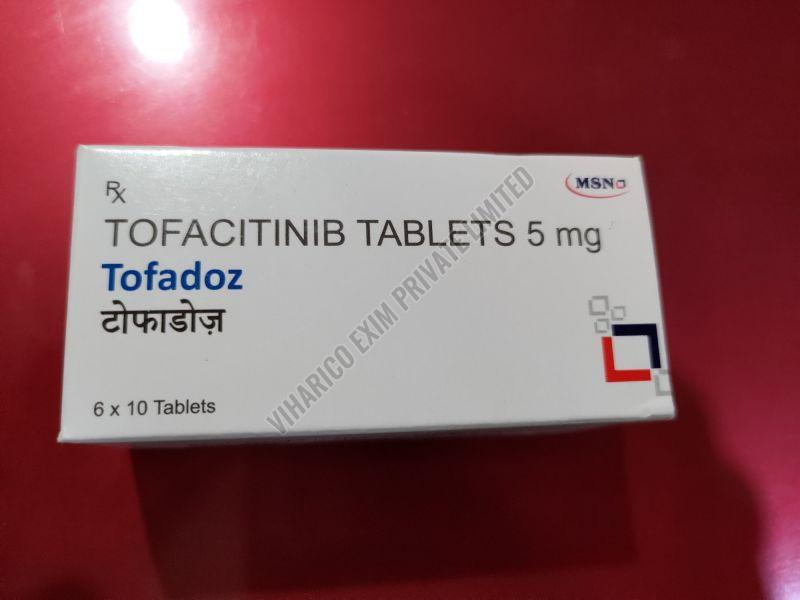
| Strength | 5MG, 10MG, 11MG |
| Shelf Life | 36 Months |
| Click to view more | |
Product Details
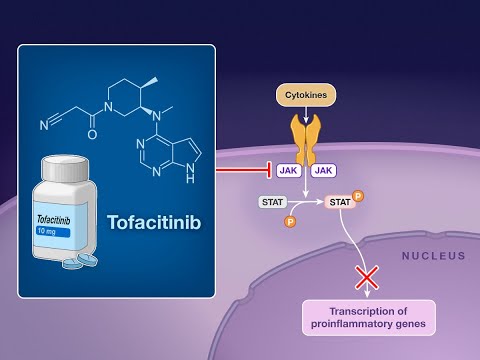
- Educational videosexplaining the mechanism of action of tofacitinib, often using diagrams to illustrate how it inhibits JAK enzymes to reduce inflammation.
- Patient information videosthat cover the uses of tofacitinib for conditions like rheumatoid arthritis and ulcerative colitis, and how the tablets are taken.
- Research summary videosthat present findings from clinical studies, which may focus on the efficacy and safety of tofacitinib compared to other treatments or the risks of cardiovascular events.
- Medical and educational channels: from reputable institutions and doctors.
- Pharmaceutical manufacturer channels, such as Pfizer, which may provide videos explaining their product's mechanism of action.
- Governmental or healthcare organization websites: that link to educational videos.
- "Tofacitinib mechanism of action" to learn how it works
- "Tofacitinib uses and side effects" for an overview of the drug
- "XELJANZ mechanism of action" (XELJANZ is a brand name for tofacitinib)
- "Tofacitinib safety" to find information on risks and warnings
Tofacitinib is FDA approved for the treatment of moderate to severe rheumatoid arthritis (RA), psoriatic arthritis (PA), ulcerative colitis (UC), and polyarticular course juvenile idiopathic arthritis (pcJIA). It is a second-generation selective Janus kinase (JAK) inhibitor targeting the JAK1 enzyme.
Tofacitinib is a prescription medication called a janus kinase (JAK) inhibitor. It is a disease modifying anti-rheumatic drug (DMARD), which works by suppressing the immune system. Tofacitinib is available in the form of a tablet (Xeljanz), an extended release tablet (Xeljanz XR) and as an oral solution.
It is used to treat certain inflammatory conditions in people who have already tried tumor necrosis factor (TNF) blockers.
When tofacitinib was approved by the FDA in 2012, it was the first approved JAK inhibitor for the treatment of rheumatoid arthritis and also the first new oral DMARD to be approved for the condition in more than a decade.
What is tofacitinib used for?
- Tofacitinib tablets and tofacitinib XR are used to treat adults with moderately to severely active
Looking for "Jakura Tofacitinib Tablets" ?
Explore More Products
Our Blogs